AI Research Assistant for Better Clinical Protocol Development
Upload your clinical protocol and instantly get insights to support your clinical development in any stage.
Trusted by researchers at
What We Do
AIRA is like a supercharged team member - fast, tireless, and always up to date.
It reviews and drafts at lightning speed, anchoring each recommendation in clear rationale and trusted sources, so you clear approvals sooner and deliver therapies to patients faster.
Know every guideline and study by heart, instantly bring the most relevant evidence into the conversation
Proactively suggests improvements, clearly explaining trade-offs and the reasoning behind each idea
Sees your entire protocol and all study docs at once, flagging gaps and inconsistencies before you even notice
Answers questions instantly and updates documents based on your instructions, making iterations effortless
Smart Reforms, Swift Decisions


Insights that Matter to You
Backs each insight with clear rationale and sources
Review literature and similar studies
Extracts relevant findings and information from other studies and publications to support knowledge discovery & identify blind spots
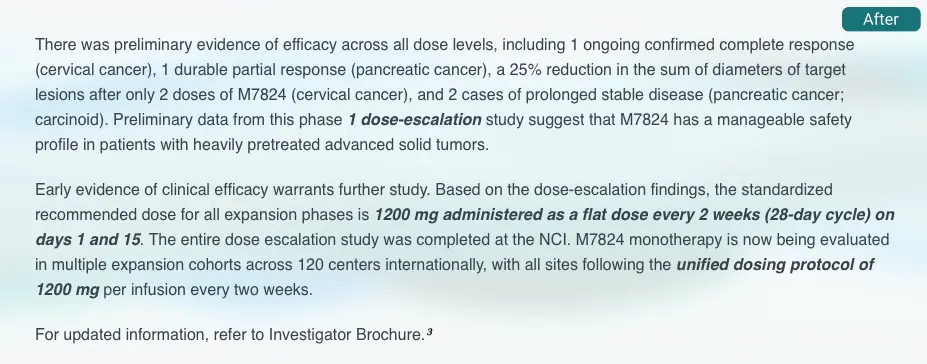
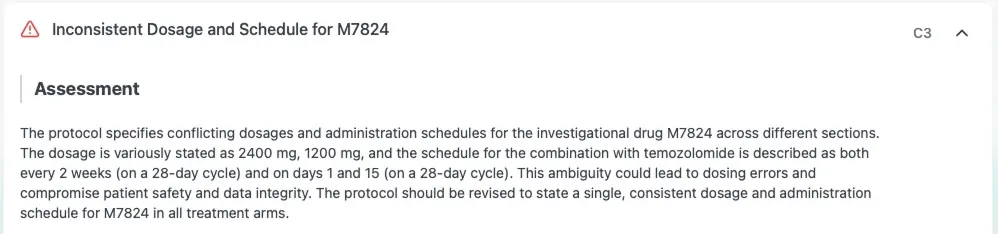
Check internal consistency
Flags contradictions and inconsistencies to reduce amendments, prevent delays, speed reviews, & ensure protocol clarity
Suggest optimization ideas
Identifies opportunities to streamline execution, improve patient & site engagement, reduce amendment risk, and align the protocol with operational goals
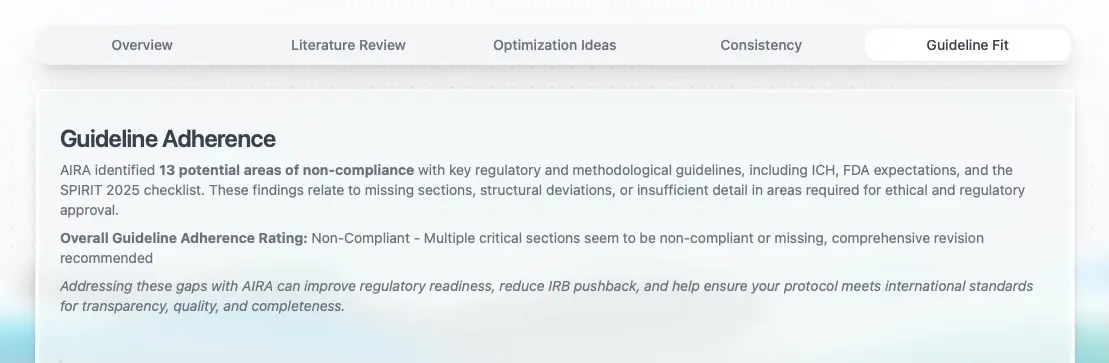
Analyze guideline adherence
Detects deviations from regulatory & methodological guidelines to help minimize IRB and agency pushback
What Researchers Are Saying
Principal Investigator
NCI-designated Cancer Center
"This is very impressive, AIRA will give back 50% of my time because I won't have to deal with the tedious work"
Clinical Research Lead
Biotech Startup
"What used to take our team weeks of literature review now happens in hours. AIRA's insights helped us optimize our patient stratification strategy."
Research Assistant
Pharmaceutical Company
"The regulatory compliance checks are incredibly thorough. AIRA identified potential FDA concerns before we even submitted our IND application."
Ready to Design Better Clinical Trials?
Join the waitlist for early access to AIRA Health. Be among the first oncology-focused biotechs to transform your protocol design process.
MVP launching August 2025 • Limited beta spots available